VIALEX is able to reverse the surface effects from the vial converting process,
resulting in a minimized risk of interaction between drug product and vial surface !
Benefits
Unparalleled Surface Quality
- Decreased risk of delamination
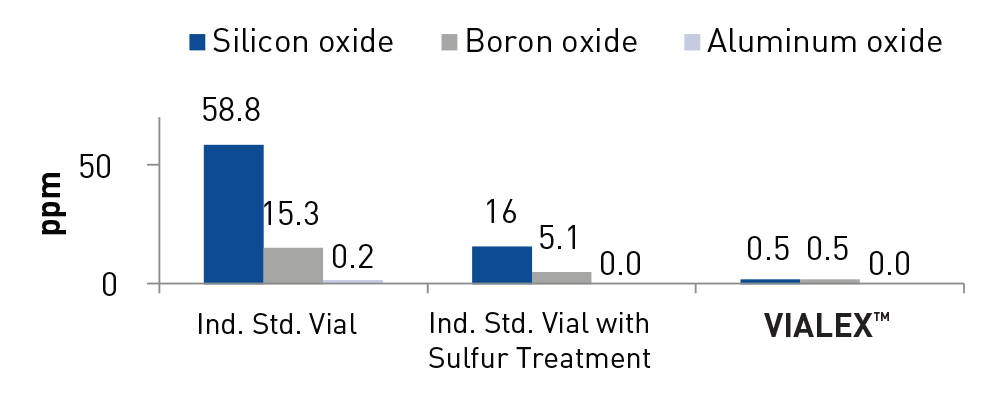
- Reduction of extractables
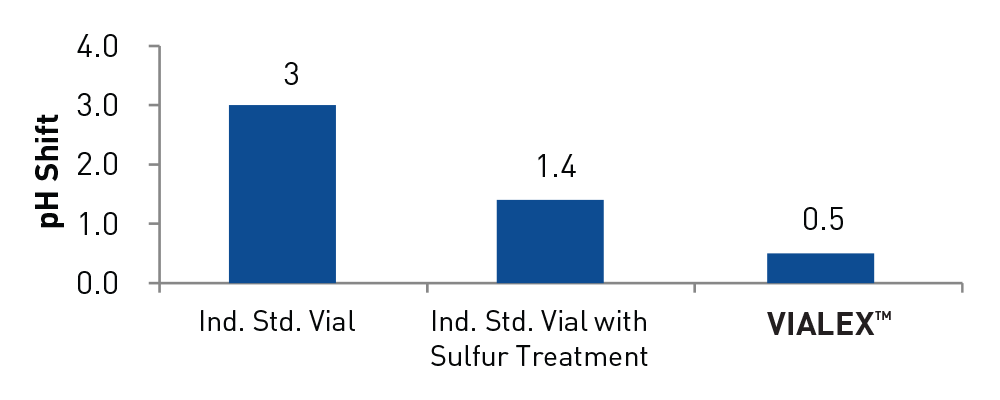
- Minimized pH shift
Easy Replacement
- No revalidation work required
- No sophisticated coatings
- No changes to glass formulation
Unique Compliance
- No risk associated with statistical sampling
- Every single vial 100% All-Points-In compliant
Proven Technology: Successfully passed over 100 challenging tests !
|
Category |
Reagent |
Cycle |
|
USP <1660> |
0.9% KCl pH 8.0 |
1 hr @ 121 °C |
|
3% Sodium Citrate pH 8.0 |
24 hr @ 80 °C |
|
|
20 mM Glycine pH 10.0 |
24 hr @ 50°C |
|
|
|
||
|
Accelerated |
Citrate @ 7.0 pH |
1 hr @ 121°C |
|
WFI @ 5-7 pH range |
||
|
0.9% NaCl @ 5.8 pH |
||
|
Acetate @ 5 pH |
||
|
Phosphate @ 7 pH |
||
|
20 mM Glycine pH 10.0 |
||
|
|
||
|
pH Rise |
0.075% KCl @ 5.9 pH for 30 min |
Varied based on customers’ specific protocols |
|
0.4% NaCl for 13 min |
||
|
0.4% NaCl for 26 min |
||
|
pH over time (KCl) |
||
|
pH over time (NaCl) |
||
Test Results
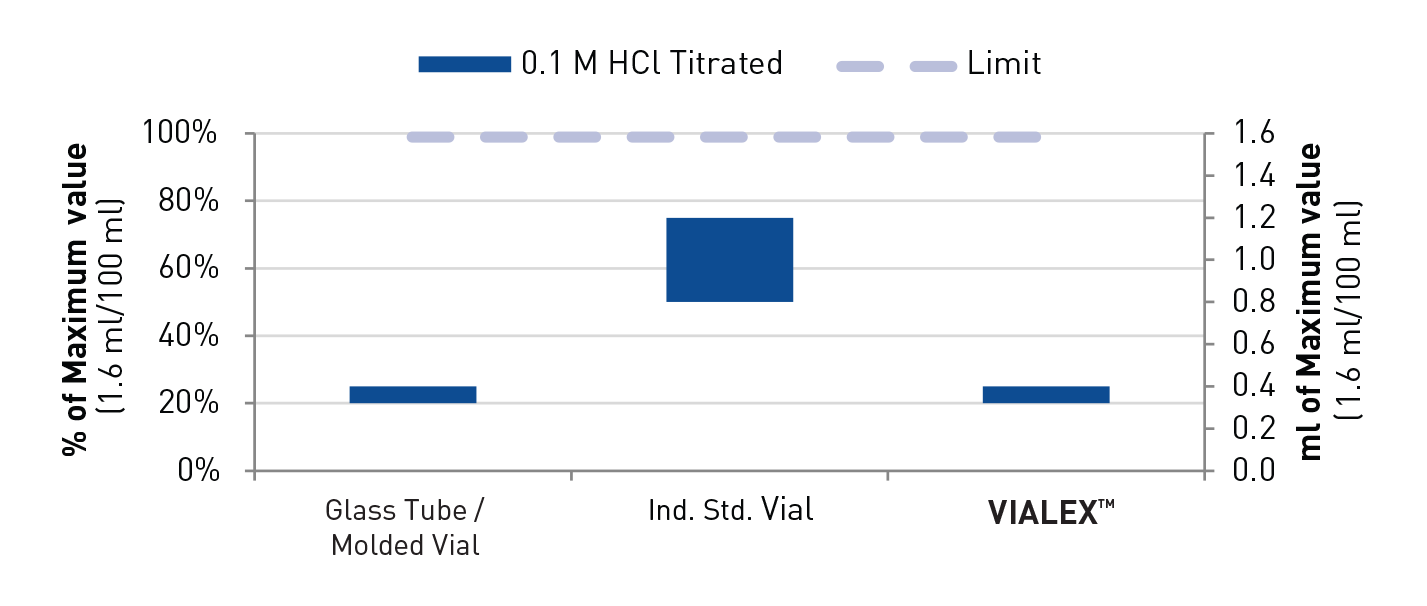
- Surface hydrolytic resistance (USP <660> EP 3.2.1 - 2 ml)
- NaCl with Terminal Sterilization at 121 °C (0.9% NaCl – pH at start 5.2 – with TS – 2 ml) - pH shift
- NaCl with Terminal Sterilization at 121 °C (0.9% NaCl – pH at start 5.2 – with TS – 2 ml) - Extractables