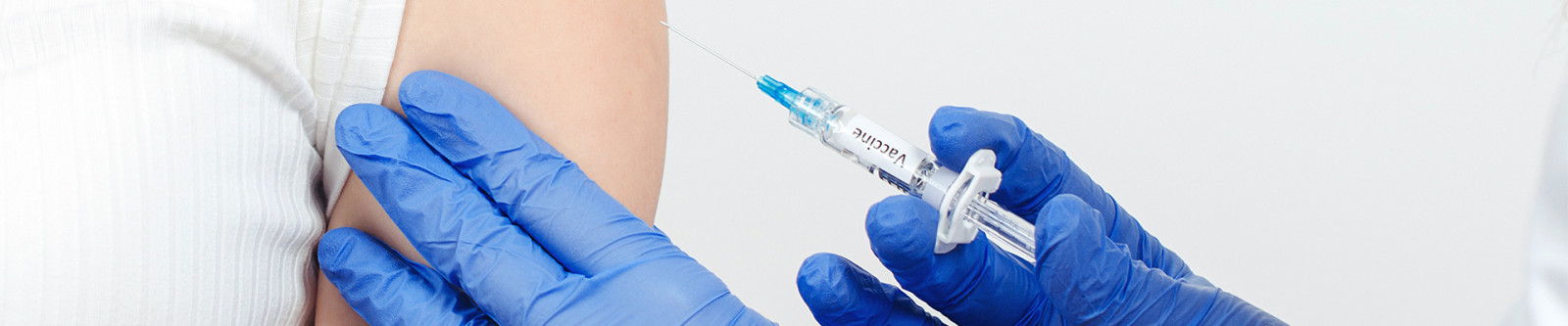
Parenteral administration of standard and advanced vaccines is essential for widespread immunization against infectious diseases. Nipro produces glass primary packaging solutions specifically designed to meet the stringent requirements for vaccines. Select from a specific range of containers tailored for vaccines or work with our multidisciplinary team of experts to develop a customized packaging solution for your pharmaceutical product.
D2F™ Pre-Fillable Glass Syringes
Excellent Drug/Container Compatibility
- Low in silicone | Silicone oil free1
[less silicone | no risk of interactions]
- Low tungsten residuals | Tungsten free2
[minimized risk of interactions | no risk of interactions]
- Minimized particle levels3
[reduced risk of interactions]
Precise Attributes for Stable Fill-Finish
-
High dimensional quality
[reliable filling and plunger fit]
-
Greater mechanical durability4
[minimized risk of breakage]
-
High cosmetic quality
[lower risk of rejection at final inspection]
-
Strong container closure integrity (CCI)5
[reduced risk of leakage]
Designed for Easier and Safer Vaccinations
-
Smooth plunger movement6
[low variability in break-loose and glide force]
-
Individual needle verification for precise penetration
[100% inspection of needle angularity and tip | needle siliconization precisely monitored]
-
Safer handling
[firm connection to standard hypodermic needles7 | proper torque and pull-off force, needle stick injury prevention system]
Advanced Service Levels and Extended Documentation
-
Data for efficient acceptance of delivered goods
[certificate of analysis | certificate of sterility | additional certificates upon request]
-
Regulatory data for standards compliance
[physical and chemical properties compliant with pharmacopeia | level of sub-visible particles | risk analysis]
-
Readily available trial samples
[helps validation of packaging, supports a fast time-to-market]
-
Drug master file data
[registered with FDA, Health Canada, and NMPA | easy procedure to receive LOA]
Recommended Syringe Range

|
For Standard Vaccines |
For Advanced Vaccines [Injection] |
For Advanced Vaccines [Intranasal]10 |
|
|---|---|---|---|
| Quality level | ENABLE | ENHANCE | ENGAGE |
|
Syringe tip |
Luer | Staked needle | LInC8 | Nasal spray |
| Syringe size | 0.5 | 1 std. | 1 long | 3 mL | 1 std. | 1 long | 2.25 mL |
150 | 200 | 250 µL |
| Closure material | FM30 | 7025/65 | 4800 GS | FM27 | FM30 | 7025/65 | 7028 | TPE |
| Stopper material | Datwyler | West | Datwyler | West | Gore9 | Datwyler | West |
| Silicone amount (mg) | 0.7 | 0.35 to 0.55 | Silicone oil free9 | Custom |
| Flange type | Cut flange | Round | Cut flange | Round | Round |
| Staked needle |
27G, ½″ - thin-wall |
n.a. | n.a. |
| Options | Backstop | Backstop | Backstop |
Footnotes
-
Amount and distribution controlled 100%, in-line
-
Only for Luer syringes
-
Use of light obscuration test for precise results, laser-based cutting technology, D2F™ process takes place in ISO 7 cleanroom under laminar air flow, syringes are washed, tub & nest are cleaned with ionized air before use, careful cosmetic inspections
-
Laser-based cutting, precise forming process
-
X-Ray inspection
-
Silicone-influenced parameters were carefully tested (break-loose force, glide force, particle level, container closure integrity)
-
ISO 11040-4 Luer cone breakage resistance compliant
-
Luer lock integrated cap
-
1 mL long only
-
EXADOSE™ Nasal Spray – D2F™ Pre-fillable Glass Syringe
D2F™ Glass Vials
Contributes to Stable Fill-finish Processes
- High dimensional quality
[for efficient fill-finish]
- Strong mechanical durability
[reduced risk of vial breakage during filling]
- Very high cosmetic quality
[lower risk of rejects at final inspection]
- Optional UID for single container tracking11
[track and trace of single containers]
Lower Risk of Drug/Container Interactions
- VIALEX™ – Significantly lower risk of interactions with the inner glass surface12
[more stable drug storage] - VIALEX™ – Reduced risk of glass delamination12
[longer shelf-life potential] - Silicone coating on inner surface of vial (optional)
[barrier between drug product and inner vial]
- Amber glass for light-sensitive medicines
- Glass tubing in ENHANCE quality
[Type I, Borosilicate, 51 expansion | high dimensional and cosmetic quality]
Enhanced Lyophilization Process
-
VIALEX™ – Optimized lyophilization process13
[reduces fogging]
Advanced Service Levels and Extended Documentation
- Data for efficient acceptance of delivered goods
[certificate of analysis | certificate of sterility | additional certificates on request]
-
Regulatory data for standards compliance
[physical and chemical properties compliant with pharmacopeia | level of sub-visible particles | risk analysis] -
Readily available trial samples
[to test packaging, supports a fast time to market]
-
Drug master file-related data
[registered with FDA, Health Canada, and NMPA | easy procedure to receive LOA]
Footnotes
11. Print of 2D data matrix code on vial barrel
12. Results of long-term study – 24 weeks at 40°C: Phosphate, High purity water, Citrate, NaCl with terminal sterilization
13. Results of Simtra Biopharma Solutions study: Introduction to Vial Fogging and Mitigation Strategies Kevin Bond, Research Associate II – Inc. Live Webinar Transcription, 2022
VIALEX™ – Exceptional Inner Surface Durability
The inner glass surface of the vial undergoes Nipro's proprietary thermal process, known as VIALEX™ treatment, which involves no additional materials or alteration in glass chemistry and is subject to 100% in-line inspection. This treatment mitigates the effects of the converting process in two ways: by reducing sodium concentrations and by improving the inner glass surface.
VIALEX™-treated vials offer unparalleled benefits for challenging drug formulations, featuring exceptional inner surface durability. The enhanced durability leads to lower levels of extractables and leachables (E&L), minimizing the risk of pH shifts and delamination events. Additionally, VIALEX™ technology has a positive effect on the lyophilization process, resulting in less fogging.
These benefits enhance the storage stability of drug products and contribute to a longer shelf-life.
Recommended Vial Range

Footnotes
14. Optional
|
For Standard |
For Advanced Vaccines |
|
|---|---|---|
| Quality level | ENABLE | ENHANCE | ENGAGE |
| Vial size (mL) | 2 R to 50 R | 2 R to 50 R |
| Glass color | Clear | Amber | Clear | Amber |
| Inner surface treatment14 | Siliconization | VIALEX™ |
| Print14 | Silk screening | Silk screening | 2D data matrix code |
| Packaging | Bulk | D2F™ | Bulk | D2F™ |
Medical Devices for Easier and Safer Reconstitution
As a vertically integrated company, Nipro offers a selected range of medical devices. D2Mix™ reconstitution devices enable safer and easier reconstitution of a drug product with a diluent in just a few steps. Consult the full range of devices to discover which device best fits your requirements.
High-quality Primary Packaging and Medical Device Solutions
for Vaccines!
For information regarding Nipro products, services, and resource material:
| Give us a call +32 15 263 500 |
E-mail us [email protected] |
| Submit a detailed inquiry: Contact per expertise |
View our manufacturing locations: Our global network |