Reliable Filling and Stable Storage of Drug Products
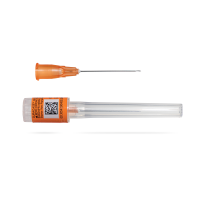
As a premier producer of high-quality pre-fillable glass syringes designed for the pharmaceutical industry, Nipro provides precise solutions tailored to meet the specific requirements of standard and complex drug products. Whether it is for vaccines, biologics, or other sensitive drug products, Nipro’s D2F™ pre-fillable glass syringes offer a trusted solution that pharmaceutical companies can rely on.
Drawing upon extensive expertise in glass manufacturing, Nipro delivers syringes that seamlessly integrate into the intricate workflows of pharmaceutical production. They contribute to smooth fill-finish operations, stable storage and smooth administrations.
Your syringes are manufactured in a state-of-the-art plant which is controlled by strict quality systems, encompassing ISO 15378, ISO 9001, ISO 45001, ISO 14001, and IS0 50001 certifications.
Our comprehensive support infrastructure, including regulatory guidance, technical assistance, and laboratory facilities, promptly attends to queries and ensures successful partnerships.
Depending on your drug product requirements you can choose from three distinct quality levels. Each quality level offers specific benefits and lists a comprehensive scope of product and service attributes that forms the perfect base to fine-tune the quality precisely to your needs.
ENABLE | Standard-quality
[Preset, standardized product specifications, Standard AQLs, Standard service level, Standard support, Standard data package]
For drug products that require standard quality and service requirements.
Optimized Processability
-
Strong CCI1 [in-line inspection of exact tip dimensions and correct position & proper POF2 of SNS3/RNS4]
-
Increased mechanical durability [high quality of glass tubing ensures precise wall thickness and exact inner/outer diameter of syringe body | in-line inspection of precise flange geometry]
-
Reliable entrance of tub onto filling line [in-line inspection of the inner diameter, flange dimensions, insert present in the tub, and nests are full and intact]
Reliable and Safer Manual Administrations
- Smooth plunger movement performance [in-line inspection of homogeneous silicone amount from flange to tip | use of dive-in nozzle]
- Controlled needle quality facilitates easy penetration [in-line inspection of needle angularity & point, needle siliconization, penetration force, and free cannula passage]
- Safer handling of syringes during manual injection [needle POF2 verified by 100% IPC | in-line inspection of accurate printing of scale/ring and SNS3/RNS4 removal force]
Standard Data Package for Fast Acceptance of Goods
-
Fast acceptance of delivered goods is facilitated [by CoC5/limited CoA6 | CoS7 | ETO-sensitive indicators on each tub & outer carton]
-
Regulatory data confirms compliance with standards [physical & chemical properties | compliant to pharmacopeia | level of sub-visible particles | risk analysis]
-
Drug Master File related data [for FDA, Health Canada, and NMPA | easy procedure to receive LoA11]
ENHANCE | High-quality
[Preset, standardized product specifications, Exceptional strict AQLs, Advanced service level, Comprehensive support, Extended data package]
For complex drug products demanding the utmost quality and service level, particularly for biologics or other highly sensitive drug products.
Excellent Drug-Container Compatibility
- Reduced risk of interactions [controlled low level/residuals of tungsten | tungsten-free luer syringes]
- Optimized silicone amount and distribution [in-line inspection of low silicone amount, silicone distribution | use of dive-in nozzles]
- Minimized particle level [strict control of glass particles, foreign matter | batch release upon measurement of particles via light obscuration test]
Designed for Integration into Auto-Injectors and Devices
- Optimal assembly and safe AID8 usage [controlled SNS3/RNS4 concentricity and barrel bowing]
- Exact fitting into AID8 [precise syringe dimensions]
- Complete dose delivery and optimal injection time [low BLF9 and consistent GF10]
- Prevention of pierced RNS3, thus AID8 with unseen loss of CCI1 [100% X-Ray inspection of SNS3/RNS4]
- Reliable use of cap removers [control of POF2 of SNS3/RNS4 ]
- Safe operation of injection mechanisms [strong mechanical durability of flange]
Optimized Performance on Filling Line for Minimal Loss of High-Value Drug Products
- High dimensional and cosmetic quality [in-line inspection of syringe dimensions (incl. consistent flange dimensions), cosmetic aspects and RNS4/closure intact, straight]
- Strong mechanical durability [excellent quality of NSV51 glass tubing (consistent wall thickness, precise inner/outer diameter) | laser-based cutting for increased durability of finger flanges]
- Optimal entrance of tub onto filling line [ETO indicator on tub that prevents entrance of unsterile tubs | foreign matter reduction by air-wash of nest and tub | automatic bagger that ensures integrity of breather bag]
Standard Data Package for Fast Acceptance of Goods
-
Fast acceptance of delivered goods is facilitated [CoA6 | CoS7 | additional certificate upon request]
-
Regulatory data confirms compliance with standards [physical & chemical properties | compliant to pharmacopeia | level of sub-visible particles | risk analysis]
-
Drug Master File related data [for FDA, Health Canada, and NMPA | easy procedure to receive LoA11]
ENGAGE | Custom-quality
Fully customize your product and service specifications to meet your unique, non-standard quality and design requirements. Customization is fully supported by an experienced and cross-functional team.
Footnotes
1. Container Closure Integrity | 2. Pull-off Force | 3. Soft Needle Shield | 4. Rigid Needle Shield | 5. Certificate of Conformity | 6. Certificate of Analysis | 7. Certificate of Sterility | 8. Auto Injection Device | 9. Break Loose Force | 10. Gliding Force | 11. Letter of Access/Authority
| Volume | Outer diameter | Flange - round | Flange - small round | Flange - cut |
|---|---|---|---|---|
| 0.5 ml | 6.35 mm | √ | √ | √ |
| 1 ml long | 8.15 mm | √ | √ | √ |
| 1 ml std. | 10.85 mm | √ | √ | √ |
| 1.25 ml | 10.85 mm | √ | √ | √ |
| 1.5 ml | 10.85 mm | √ | √ | √ |
| 2.25 ml | 10.85 mm | √ | √ | √ |
| 3 ml | 10.85 mm | √ | √ | √ |
| Closure | Plunger rod | Plunger stopper | Backstop |
|---|---|---|---|
| Easy-turn tip cap | Transparent | Standard | Transparent |
| Ribbed tip cap | Color | Coated | Color |
| Bagged | RTU |
Sterile Packaging Solution
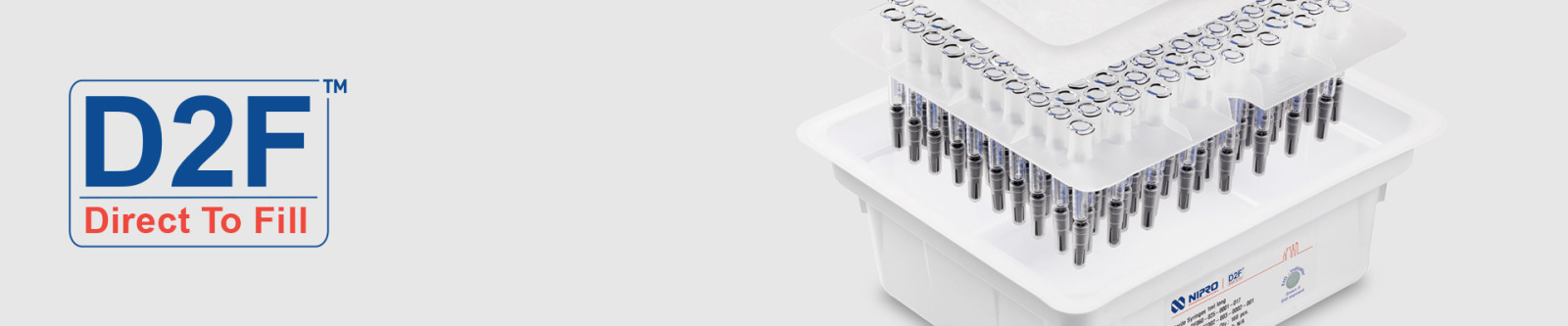
Nipro manufactures D2F™ syringes in technologically advanced production sites that are certified according to ISO 9001, ISO 15378, ISO 14001, and ISO 50001. Our fully automated cleaning and packaging process takes place in an ISO 7/ISO 8 cleanroom with 100% monitoring under laminar air flow.
Key-technologies:
- Less particles: Tub and nest are cleaned by ionized air before entering the D2F™ process
-
Optimal entrance of tub on filling line: 100%, In-line camera inspected (nest full, closure on syringe present, correct Tyvek® placement)
- High cosmetics quality for less risk of rejects at final inspection: No glass-to-glass contact in tub
- Visual control that tubs were sterilized: ETO indicator on tub (color change from blue to green after ETO exposure)
Nipro’s D2F™ packaging design is a smart, efficient choice that enables immediate and direct use on the filling line. In essence, Direct-to-Fill.
| Tub insert | Breather bag | Outer box | Pallet | Slip sheet insert |
|---|---|---|---|---|
| Single (Tyvek) | Single | Cardboard | Wooden (treated) | Optional |
| Double (Tyvek) | Double | PP-well | Plastic |
Frequently Asked Questions (FAQs)
"PFS" stands for "Pre-Filled Syringe." A pre-filled syringe is a type of medical device used for the storage and administration of medications. It consists of a syringe barrel pre-filled with a specific dosage of medication, a plunger, and needle or needle cap. Pre-filled syringes are designed to be used immediately without requiring additional preparation or assembly by healthcare professionals.
Pre-filled syringes offer various advantages when compared to other container types:
- Accurate dosing
- Convenience and ease of use
- Reduced risk of contamination
- Improved patient safety
- Minimized medication wastage
- No reconstitution is required
Yes, silicone oil-free syringes do exist. These syringes are manufactured using materials and processes that eliminate the presence of silicone oil, which can be a concern for certain pharmaceutical applications.
For information regarding Nipro products, services, and resource material:
| Give us a call +32 15 263 500 |
E-mail us [email protected] |
| Submit a detailed inquiry: Contact per expertise |
View our manufacturing locations: Our global network |